The first retinal implant has recently been approved by the U.S. Food and Drug Administration (FDA). As reported by Reuters, this device will help replicate some of the functions of the retina that have been destroyed by retinitis pigmentosa.
The implant, called the Argus II, is composed of a special pair of glasses with a video camera and video processing unit, as well as a wireless receiver that is implanted in the eye. The entire device mimics the retinal functions that process what we see and relay it to the optic nerve. While the implant doesn’t provide total vision restoration, it can help patients with everyday activities like recognizing large letters or shapes.
The Argus II was first approved for use in Europe in 2011. Since the beginning of a clinical trial in 2007, the device has been implanted in 30 patients. Mark Humayun of the University of Southern California’s Keck School of Medicine and USC’s Viterbi School of Engineering shared with Reuters “In the patients that have been implanted to date, the improvement in the quality of life has been invaluable.”
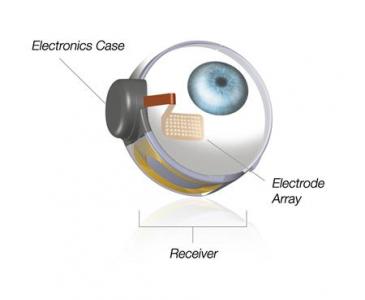
Image via Reuters.com
The news that this retinal implant has been approved by the FDA is certainly exciting, not only for practitioners but also patients who have suffered the most adverse effects of retinitis pigmentosa. This is a great advancement, but it’s also important to remember that good retinal care includes annual comprehensive exams with ultra-widefield imaging. Optos offers several ultra-widefield imaging devices that offer a pain-free exam for patients, providing the widest view (up to 200°) of the retina in one single capture and the ability to detect and diagnose issues that could be affecting a patient’s vision much sooner than with conventional equipment.
Visit the Optos website to learn more about our ultra-widefield retinal imaging devices and how they can benefit your practice and your patients. You may also request a consultation with an optomap professional for further information.