Age-related macular degeneration (AMD) is a leading cause of blindness in the United States, affecting most commonly, people over the age of 60 with increasing chances as you age, if you are overweight or if you have a family history of AMD.
As we approach our golden years, we are at a higher risk for particular eye diseases including age-related macular degeneration, cataracts, diabetic retinopathy, glaucoma, as well as eye conditions such as dry eye and low vision. More than 40 million Americans are currently 65 years or older, this number is expected to grow to more than 88 million by 2050 and not coincidentally, the number of Americans with age-related eye diseases is expected to double. Early detection and treatment are key to saving sight.
For some, AMD advances so slowly that vision loss does not occur for a long time. In others, the disease progresses faster and may lead to a loss of vision in one or both eyes. The loss of central vision in AMD can interfere with simple everyday activities, such as the ability to see faces, drive, read, write, or do close work, such as cooking or fixing things around the house.
As the disease progresses through the asymptomatic phase, it moves from Dry AMD to Wet AMD. In geographic atrophy (dry AMD), there is a gradual breakdown of the light-sensitive cells in the macula that convey visual information to the brain, and of the supporting tissue beneath the macula. In neovascular AMD (wet AMD), abnormal blood vessels grow underneath the retina. These vessels can leak fluid and blood, which may lead to swelling and damage of the macula. It is important to assess the risk of progression from the dry type to the wet type of the disease.
Although there have been many discoveries in the understanding of the causes of AMD, including links to genetics, there remains much unknown about this complicated, degenerative disease. With the advent of multi-modality ultra-widefield (UWF™) imaging, the retinal periphery has been able to be more easily studied in AMD to determine the value in the detection and/or monitoring of the disease. Color optomap® imaging captures the structure and fundus autofluorescence (FAF) the function, of the Retinal Pigment Epithelium (RPE) which is where AMD manifests within the eye. Recent studies have revealed that 97% of patients with AMD have evidence of the disease outside of the central pole. This outcome demonstrated that drusen and reticular changes were seen in a majority of eyes, strongly indicating that AMD is more than a macular condition but one that involves the entire retina. This is being investigated in a further study that will determine whether these peripheral changes are associated with the progression of the disease.
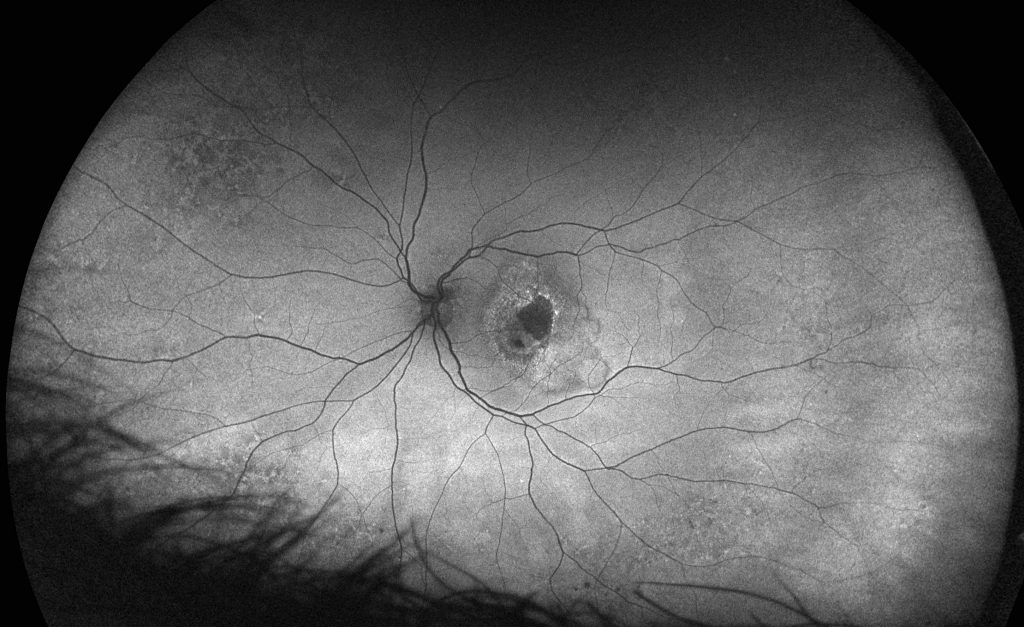
Glaucoma is another leading cause of irreversible blindness in the US and around the world with more than 3 million Americans living with the disease, 2.7 million of them over the age of 40. While early detection is key to taking steps to prevent vision loss, glaucomatous vision impairment is irreversible. Unfortunately, glaucoma can be asymptomatic until the late stages, at which time the prognosis is poor.
The gold standard for detection and diagnosis of glaucoma is a clinical examination with dilated slit lamp biomicroscopy conducted by a glaucoma specialist. However, this level of expertise is not always feasible or readily available to broadly evaluate an aging population. Exam efficiency has been increasingly addressed via use of color digital stereoscopic photography and/or retinal tomography via SD-OCT.
A recent study explored the potential suitability of ultra-widefield retinal imaging in supporting the diagnosis of glaucoma in situations where slit-lamp biomicroscopy or digital color stereoscopy are not available. The purpose of the study was to evaluate the reproducibility and validity of UWF in estimating Vertical Cup to Disc Ratio (VCDR) measurements and was the first study of its kind to explore whether optomap imaging could be suitable as a diagnostic support tool for glaucoma.
The study evaluated the data from color digital stereoscopic fundus images (CDS) and UWF images. All the photographs and images were graded by two masked trained graders and one masked glaucoma specialist. The optomap images were graded using the ‘measure distance’ tool on the OptosAdvance™ software, to measure and record cup to disc ratio (CDR).
The study evaluated the data from color digital stereoscopic fundus images (CDS) and UWF images. All the photographs and images were graded by two masked trained graders and one masked glaucoma specialist. The optomap images were graded using the ‘measure distance’ tool on the OptosAdvance™ software, to measure and record cup to disc ratio (CDR).
The study demonstrated an almost perfect agreement between CDS and optomap when assessed by the glaucoma specialist. The study concludes that optomap imaging has a high reproducibility in evaluating VCDR and agreement with stereoscopic optic disc imaging and indicates that UWF imaging may be suitable for glaucoma evaluation in settings where CDS is not available.
Visit our website to review additional clinical studies and learn more about utilizing sight-saving optomap technology.
https://nei.nih.gov/nehep/ham
https://www.brightfocus.org/glaucoma/article/glaucoma-facts-figures